
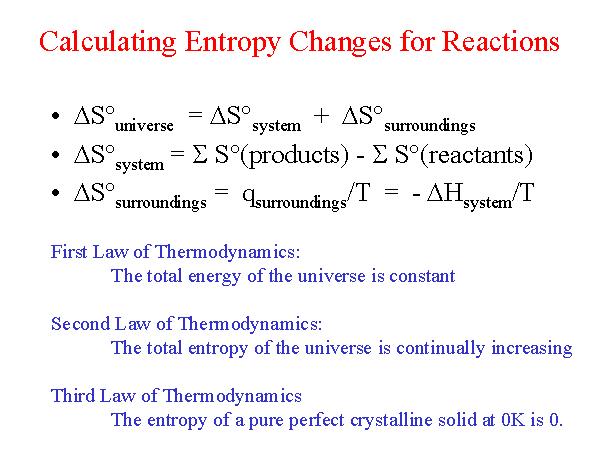
The increase of disorder provides most of the free energy. ΔH The free energy comes mostly from a flow of thermal energy. ΔH > T * ΔS then the reaction is enthalpy-driven.The direction of a free energy change can be either enthalpy- or entropy-driven. ΔG0 - a nonspontaneous process - additional energy must put in for the reaction to happen (a round boulder being pushed up a hill).We can also define it with regards to the change in free energy: The Gibbs free energy equation is:Įarlier, we talked about spontaneity of a process and how it is associated with entropy. It's a function of both enthalpy and entropy, and is used to predict the spontaneity of a processes. What is Gibbs free energy? It's the energy in a system available to do work on its surroundings at constant pressure and temperature. Every system tends toward stability, and, for an irreversible process, maximum stability is reached it when the system's energy is most disordered. The entropy change of solids and liquids can be obtained by applying the above two equations to the first Tds equation. The entropy of a system is strictly connected to the systems energy. As stated by a physicist Rudolf Clausius: "The entropy of the universe tends to a maximum." You intuitively know that the opposite process is not possible - the milk won't separate from coffee by itself.Īny spontaneous process increases the disorder of the universe. You observed that the milk quickly mixes with the coffee. Let's say you've made yourself a hot cup of coffee. It goes from high pressure on one side of the valve to low pressure on the other side, while the temperature change is to some degree positive(zero).
It might sound complicated, but you'll understand it easily with an everyday example. Answer (1 of 3): In an adiabatic throttling process, the enthalpy change is zero. It doesn't have to be a fast - it can even be still occurring when the heat death of the universe occurs - but if it would proceed without the addition of any outside energy, it's spontaneous. The formula DSDQ/T is applicable only if a system undergoes a quasi-static. The system (the water) will decrease in entropy first because its. It's one of the main determinants of the spontaneity of a reaction.Ī spontaneous process is one that doesn't require an outside source of energy to proceed. I confused about change of entropy in free expansion in adiabatic condition. Water freezing in a constant temperature surroundings at -5 C is an example of this. But why measure disorder, and is it even possible? Physically, we can't measure entropy, but we can calculate it.

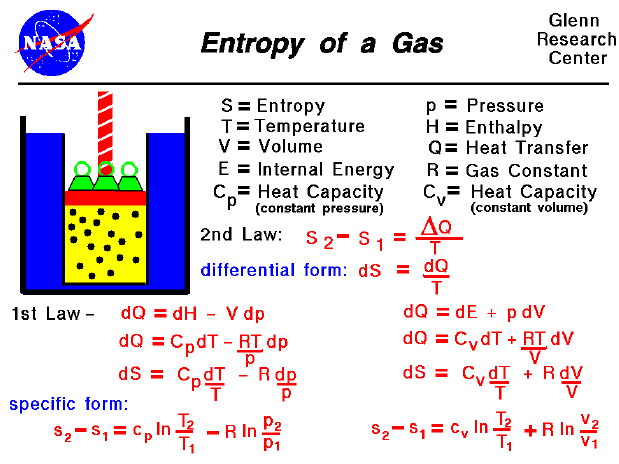
The disorder of a system is always increasing.Įntropy is the measure of that disorder. The second law of thermodynamics states that:


 0 kommentar(er)
0 kommentar(er)
